-
Sialidase Au A-(2-3,6,8,9)
-
需要定制不同规格吗?请联系我们。
α(2-3,6,8,9) Sialidase Au cleaves all cleaves all non-reducing terminal sialic acid residues from complex carbohydrates and glycoproteins. The relative cleavage rates for different linkages are:α(2-6) >α(2-3) >α(2-8),α(2-9). In addition, the enzyme will cleave branched sialic acids (linked to an internal residue). This property makes it unique among sialidases. High concentrations of enzymes and prolonged incubation times may be required for cleaving branched residues. To cleave only non- reducing terminalα(2-3) unbranched sialic acid residues, use Sialidase SP, part numberE-S007. α(2-3,6,8,9) Sialidase Au is isolated from a clone of Arthrobacter ureafaciens. The enzyme has been extensively characterized using oligosaccharide standards.
-
-
-
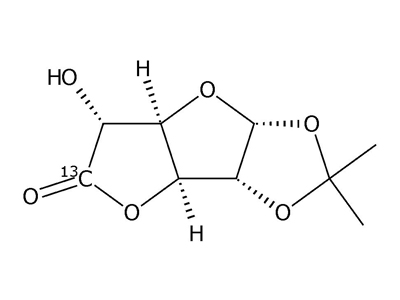
1,2-O-Isopropylidene-β-L-idofuranuronic-6-13C acid γ-lactone
-
-
-
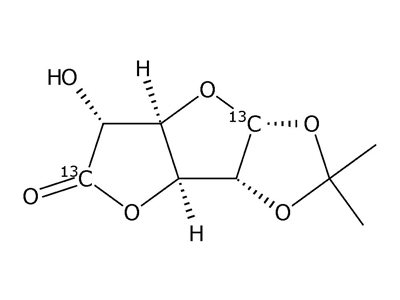
1,2-O-Isopropylidene-β-L-idofuranuronic-1,6-13C2 acid γ-lactone
-
-
-
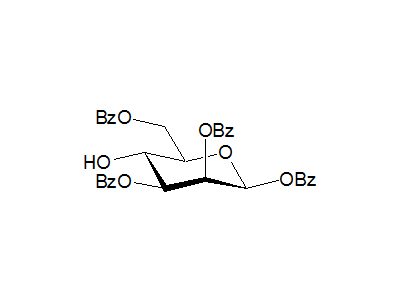
1,2,3,6-Tetra-O-benzoyl-β-D-mannopyranose
-
-
-
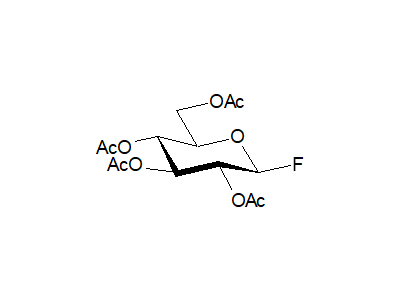
2,3,4,6-Tetra-O-acetyl-β-D-glucopyranosyl fluoride
-
-
-
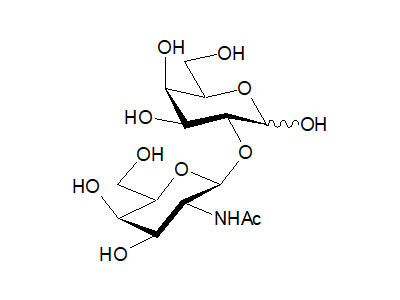
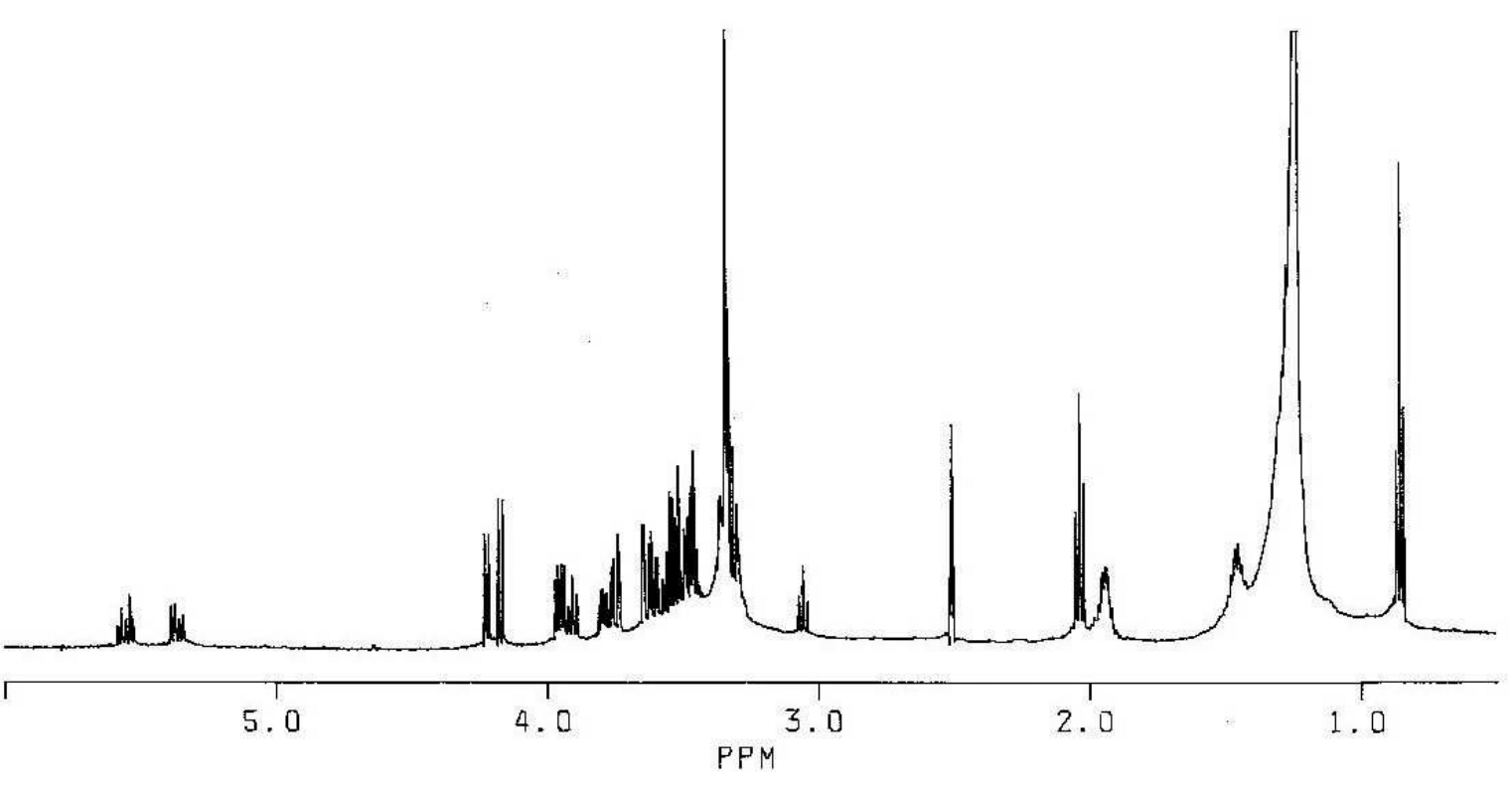
2-O-(2-Acetamido-2-deoxy-β-D-galactopyranosyl)-D-galactose
-
-
-
Lactosylceramid
-
-
-
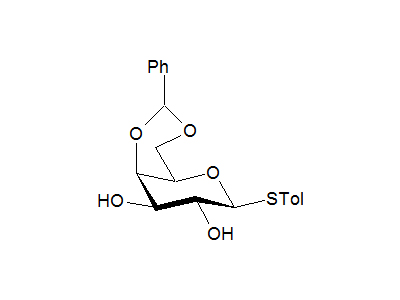
4-Methylphenyl 4,6-O-benzylidene-1-thio-β-D-galactopyranoside
-
-
-
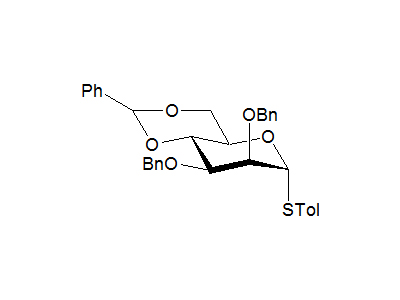
4-Methylphenyl 2,3-di-O-benzyl-4,6-O-benzylidene-1-thio-α-D-mannopyranoside